The TIPTOE Study: MulTI-domain Self-management in Older People wiTh OstEoarthritis and Multi-Morbidities (TIPTOE)
We would like to invite you to take part in our research which is studying a new treatment support designed to help individuals living with knee and/or hip joint pain and other long-term conditions better manage their health and well-being in everyday life.
Joining the study is entirely up to you, before you decide we would like you to understand why the research is being done and what it would involve for you. Please take the time to read the following information carefully and discuss it with others if you wish. Throughout this information sheet, the use of the term ‘we’ refers to the TIPTOE study team and Sponsor (Cardiff University).
The first part of the Participant Information Sheet tells you the purpose of the study and what will happen to you if you take part. Then we will give you more detailed information about the conduct of the study. Do ask if anything is unclear.
This page highlights the main points in taking part. Please read the rest of the information sheet for more details.
To establish whether, compared to treatment normally offered (‘usual care’), treatment with a new living well support can help individuals with knee and/or hip joint pain and other long-term conditions.
Because you might be eligible to take part in the study. We will not be able to confirm this until you have answered a few questions.
It is up to you to decide whether or not you take part.
Either you will continue to receive your ‘usual care’ or you will receive a new treatment which we call the TIPTOE living well support. This will involve up to 6 one-to-one sessions with a healthcare practitioner over a 6-month period to help you better manage your knee and/or hip joint pain and other long-term condition(s). You will also have access to resources such as a specially developed book with information and tips for living with your long-term condition. We will also ask you to complete questionnaires at the start of the study and after 6 and 12 months. Your involvement in the study will last for one year in total.
The study is being run by Cardiff University, in collaboration with other universities (Swansea University, St. Georges University of London, the University of Bournemouth, the University of Plymouth). Our other partners are Bridges Self-Management, a social enterprise with specialist expertise in living well treatments and Baseline Solutions, a provider of websites and electronic systems. Bridges Self-Management have worked with us and a range of older people living with joint pain and other medical conditions to develop the TIPTOE living well support and the training for staff delivering this treatment. Baseline Solutions are creating the study website where you can register your interest in the study and where you can complete the study questionnaires.
Neither you nor the study team will have any influence over whether you receive the TIPTOE living well support or continue with your usual care. This decision will be made by a computer on the basis of chance, that is random, like flipping a coin.
We are running this study because we do not know if the new TIPTOE living well support is better than usual care so taking part may not be of direct benefit to you. It should, however, help us to provide better care for others in the future.
Please see the diagram below, giving an overview of the study process. More detailed information about what is involved can be found later on in this section of this participant information sheet.
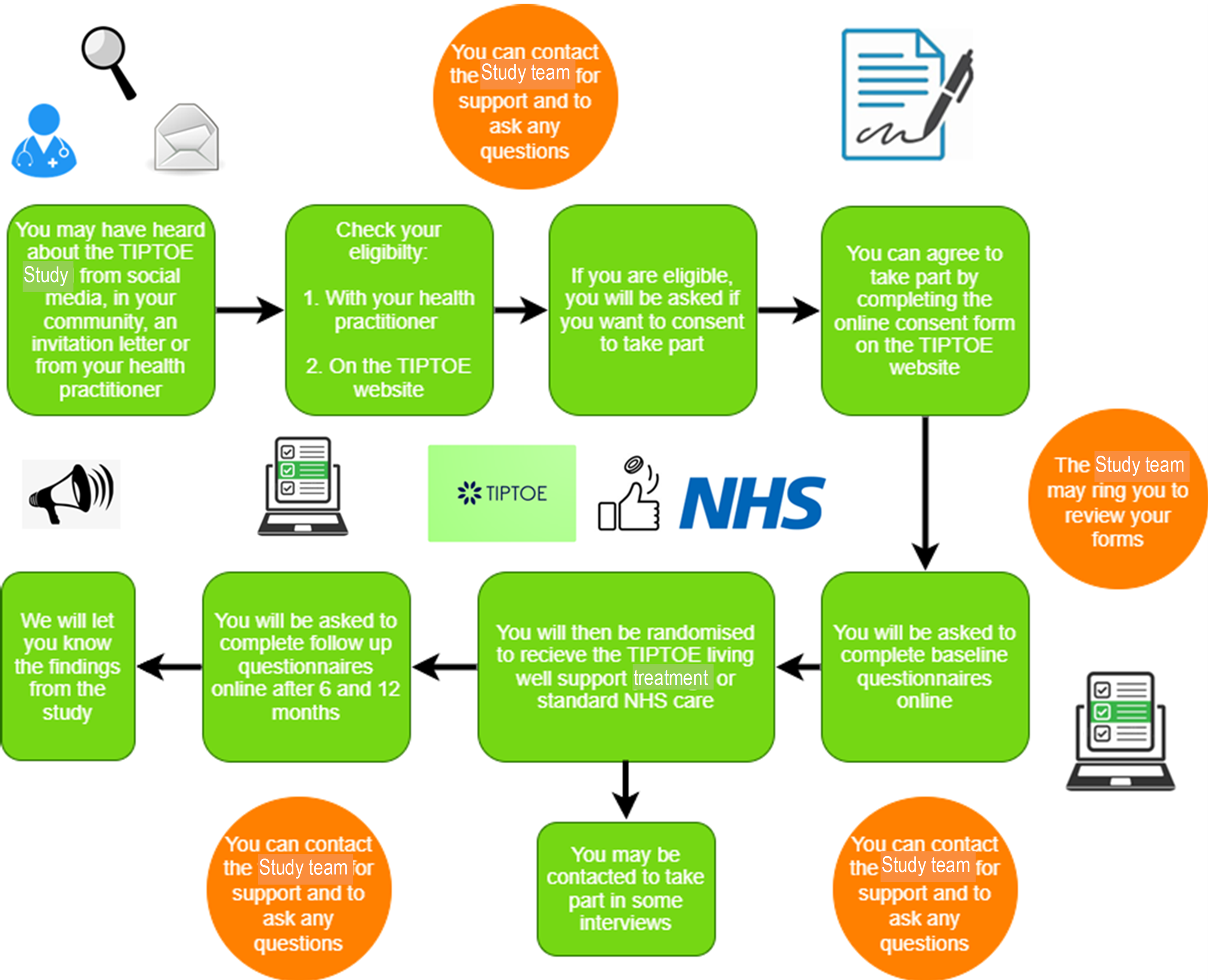
People with knee and/or hip joint pain are more likely to also have other long term health conditions which may mean they have less independence and have a lower quality of life in terms of mobility, pain, anxiety and depression. This can be made worse when healthcare professionals are unclear about how best to care for people with multiple long-term conditions.
The TIPTOE team and Bridges Self-Management have worked with individuals living with knee and/or hip joint pain and other long-term conditions to design a new personalised support treatment which we call the TIPTOE living well support. This includes one to one support sessions and useful resources. This study will evaluate how effective this new TIPTOE living well support is and see whether it can help individuals with knee and/or hip joint pain and other long-term conditions understand their conditions more and be able to cope with the challenges they experience in everyday life.
We are looking for 824 people with knee and/or hip joint pain and at least one other long-term condition to take part in the study. The study will use a randomised design. By randomised, we mean that if you agree to take part you will be randomly allocated to one of two groups by a computer programme. Either you will receive your usual NHS care OR the TIPTOE living well support for 6 months in addition to your usual NHS care. The choice of group is based entirely on chance. We will use a computer system to decide whether you receive the TIPTOE living well support or continue with your usual NHS care.
You are receiving this information as you have knee and/or hip joint pain and are living with another long-term condition and you have indicated that you are happy to be given more information about the TIPTOE study.
You are eligible to take to part in the study if:
You are not eligible to take part in the study if:
If you are interested in taking part, you can check if you are eligible by completing eligibility questions on the study website. If you are unsure of your eligibility or have any queries, please contact the study team. Contact details are available on the study website, and at the end of this information sheet. If you are invited to take part in person, then your healthcare professional may guide you through the eligibility process. We encourage you to identify someone close to you (e.g., a family member, friend or carer) who can act as a nominated supporter to help you to take part in the study.
If you are eligible, and want to participate in the study, you will be asked to complete an informed consent form before any data collection can start. You will then be asked to complete several questionnaires about your knee and/or hip joint pain and other long-term condition(s) and how you are coping with them. We would like you to complete all questions, and can be supported by your nominated supporter (if you have one). You can complete all forms and questionnaires online, by telephone or in person with your healthcare professional. If you complete the informed consent form online, telephone support will be available for you. Once you have completed the questionnaires, you will be randomly allocated to receive either the new TIPTOE living well support or to carry on with your usual NHS care. Someone will contact you to discuss this.
If you are offered the TIPTOE living well support, you will receive up to 6 one-to-one sessions with a healthcare practitioner. These may take place in person, by telephone or on an online platform such as Zoom or Microsoft Teams, depending on availability and your preference. These sessions will take place over a 6-month period to help you self-manage your knee and/or hip joint pain and other long-term condition(s). You will also have access to resources such as a specially developed book called ‘Living Well with Joint Pain’. This has information and tips for living with your long-term condition. The book will be sent to you in the post by the Bridges living well team.
Physical activity monitor
Everyone taking part will be asked if they would be willing to use an activity monitor. Not everyone will be sent a monitor as we only have a limited number of them. Therefore, we will only send one if you have agreed to it and we have one available. If you are sent a physical activity monitor, we ask that you wear it for 7 days at the beginning of the study, and again 12 months later. The photo below gives you an idea of what the monitor looks like and where to wear it, but we will give you full instructions when we send it to you. You can also give us a call if you are not sure what to do. The activity monitor is approximately 2.5cm wide, 4.3cm long (about half the length of the long edge of a credit card) and 0.5cm thick.

During the first 6 months, you (and your nominated supporter, if appropriate) may be invited to take part in up to 3 interviews with a researcher using an online platform (e.g. Zoom or Teams) or by telephone, depending on what is most convenient for you. The purpose of these interviews is to capture your experiences of taking part in the study.
After 6 months of being in the study, we will ask you to complete the questionnaires again, and to repeat these after another 6 months to let us know what health services you have used and your quality of life. The healthcare professionals or someone from the TIPTOE study team will remind you when it is time to complete the questionnaires. We expect that filling out those forms will take approximately 30-60 minutes in total to complete. You do not need to complete them all at once. You can take breaks as needed.
If you complete both your 6 and 12 month follow up questionnaires, you will be given the opportunity to be entered into 2 separate prize draws to win £100 worth of vouchers. Those participants who take part in interviews will be given a £10 voucher per interview.
If during the 12 months when you are participating in the study you are (1) admitted to hospital for any reason, (2) your knee and/or hip joint pain symptoms get substantially worse or (3) there is any other incident you feel relevant that you haven’t previously told us about, please let us know either via the website or by telling your local study healthcare practitioner. If there are any concerns about your health we may need to let your GP know.
At this stage of the research, we don’t know if there will be any direct benefits for you but by being involved you will help us gather evidence about different ways that may help individuals with knee and/or hip joint pain and other long-term condition(s) manage better in everyday life.
There is a chance that you might find some topics sensitive or challenging whilst taking part in one-to-one support sessions or whilst answering questionnaires. If you do experience any serious adverse effects while participating in the TIPTOE study, we would like you to let the study team and your healthcare practitioner know. We will tell you how to do this at the start of the study.
You can express interest in several ways:
No. It is your choice. If you do decide to take part but change your mind, you can withdraw from the study at any time, without giving a reason. Not taking part, or deciding to withdraw, will have no impact or influence on your healthcare. If you choose to take part and consent, the study team will send a letter to your GP to let them know of your involvement and what the study involves.
If you participate and then decide you want to withdraw from the study, the information we have recorded about you whilst you were on the study may still be used unless you request for your data to be destroyed. Please note that in some circumstances, it will not be possible to destroy your data. For example, if the analysis has already been conducted or if reports/publications have been written. All safety reports will also need to be retained.
If you lose capacity to be able to continue to consent to taking part in the study, we will seek to approach someone who knows you well, or cares for you to provide advice about your continued participation. This might be your nominated supporter who could be asked to act as a consultee. If the consultee agrees for your participation to continue, we will continue the TIPTOE support and data collection as usual. If the consultee advises against your continued participation, you will be withdrawn from any further participation in the study and no further data will be collected. Data collected while you were able to consent would be kept and used in the study. Additionally, if any information becomes available to the TIPTOE study team that may be relevant to your willingness to continue with participation in the study, you will be informed in a timely manner.
Results from the study will be published in scientific journals and also presented at relevant conferences. We will also share results with all study participants. All study results presented to the public will be made anonymous so that you cannot be identified from the data.
The study is being sponsored by Cardiff University and the Centre for Trials Research at Cardiff University is managing the study on a day-to-day basis. The study is funded by the National Institute of Health and Care Research. We have worked extensively with a Patient and Public Involvement (PPI) group who have helped us shape the study.
The study has been approved for conduct in the NHS from a Research Ethics Committee (REC) that is legally ‘recognised’ by the United Kingdom Ethics Committee Authority for review and approval.
If you have a concern about any aspect of this study, you should ask to speak to the study researchers who will do their best to answer your questions TIPTOE@cardiff.ac.uk .
If you remain unhappy and wish to formally complain, you can do this by contacting:
Dr Rachel McNamara
Director of Brain Health and Mental Wellbeing,
Centre for Trials Research,
Neuadd Meirionnydd,
Heath Park
Cardiff
CF14 4EP
Tel: +44 (0)29 2068 7614
Email: mcnamara@cardiff.ac.uk
If you have any concerns about our use of your personal information, you can make a complaint to Cardiff University as study Sponsor at inforequest@cardiff.ac.uk
You can also complain to the ICO if you are unhappy with how we have used your data.
The ICO’s address:
Information Commissioner’s Office
Wycliffe House
Water Lane
Wilmslow
Cheshire
SK9 5AF
Helpline number: 0303 123 1113
ICO website: https://www.ico.org.uk
In the event that something does go wrong, and you are harmed during the research, and this is due to someone’s negligence then you may have grounds for legal action for compensation against Cardiff University, but you may have to pay your legal costs. The normal NHS complaints mechanisms will still be available to you (if appropriate).
Thank you very much for considering taking part in this study.
The TIPTOE Team Contact Details: (Rachel Deere/Ffion Davies)
Ring us: 02920 688 303 Monday to Friday (09:00 – 17:00)
Email us at: TIPTOE@cardiff.ac.uk
TIPTOE Study Team, Centre for Trials Research
Cardiff University
4th Floor, Neuadd Meirionnydd
Heath Park
Cardiff
CF14 4YS
Versus Arthritis Get help | Helpline, online community, arthritis virtual assistant (versusarthritis.org)
We will need to use information from you for this research project. This information will include your name, contact details (including your postcode) and GP surgery. People will use this information to do the research or to check your records to make sure that the research is being done properly.
People who do not need to know who you are will not be able to see your name or contact details. Your data will have a code number instead. We will keep all information about you safe and secure.
Once we have finished the study, we will keep some of the data so we can check the results. We will write our reports in a way that no one can work out that you took part in the study.
All information will be kept private, confidential and secure. Your research data will be stored on secure servers at Cardiff University and Swansea University. If the result of the study is published, your identity will remain confidential. What you say/communicate in one-to-one sessions and interviews will be typed out by a professional transcriber to make a transcript. All identifiable information in the transcript will then be made anonymous by changing your name and locations, for example.
Your contact details will be kept securely on Cardiff University secure servers and/or locked storage facilities at Cardiff University.
The interview transcripts will be kept securely on Cardiff University’s secure servers.
Only people involved in this study or in making sure it is run correctly (e.g., auditors and regulatory authorities) will have access to your information. No identifiable information will be used in any reports.
Personal data will be securely destroyed within 3 months of the end of the study. All other non-identifiable data will be retained by Cardiff University for a minimum of 15 years.
As part of the consent process, we will also ask your permission to securely send some personal information collected from you as part of the study (name, date of birth, gender, NHS number) to NHS England (if you live in in England) or Secure Anonymised Information Linkage (SAIL) (if you live in Wales), who hold information about your NHS health records such as primary care data, hospital admissions, outpatient appointments and emergency care admissions.
The purpose for us sending your information to these organisations is so that they can match your details to some of your NHS healthcare records (for the six months before, during and 12 months after your involvement in the study) and make it available to the research team to allow us to look at how often people in the study use NHS health services.
The healthcare information that the research team will receive from these organisations will have all identifiable information removed (it won’t contain your name, post code or other personal information) and will be limited to information that is relevant to this research project. Access to the information will be restricted to trained researchers from the study team, who will only be able to view it via a secure online system (called a Trusted Research Environment). We will not share this information with anyone else and will only have access to this information for the duration of the study, until we complete our analysis.
Accessing people’s NHS records in this way means that we don’t have to ask you to fill out lots of questionnaires that ask you to recall how often you’ve been to hospital, for example, over the last six or twelve months.
Under the General Data Protection Regulation (GDPR), the lawful basis we rely on for processing this information would be public task.
You can find out more about how we use your information: